Hunting for the Next Moderna
As a city awash in world-class hospital systems and explosive biotech firms, we’ve never been ones to rest on our laurels. Which begs the question: What’s next for Boston innovators?
Moderna and its first-of-its-kind COVID vaccine helped save the world—further cementing Bostonians as titans of innovation, and turning the investment-savvy among us into millionaires. Still, as a city awash in world-class hospital systems and explosive biotech firms, and where Harvard degrees have become a dime a dozen, we’ve never been ones to rest on our laurels. Which begs the question: What’s next? In search of an answer, we dug deep for innovations with the potential to transform lives (and wallets). Here are the 10 that impressed us most.
Edited by Andrea Timpano

Dr. Navin Kapur holds the apparatus used as part of the Door-to-Unload approach, developed at Tufts Medical Center. / Portrait by Webb Chappell
The Heart Pump
(Not so fun) fact: Every 40 seconds, someone in America suffers a heart attack. And while current interventions have proven effective, “many patients are surviving the heart attacks and leaving the hospital with damaged hearts,” says Dr. Navin Kapur, executive director of the CardioVascular Center for Research and Innovation at Tufts Medical Center.
That’s where the Impella CP comes in. A heart pump developed by Danvers-based medical technology company Abiomed, the Impella is inserted into the heart 30 minutes before the start of an angioplasty—the most widely used heart attack treatment, which employs a balloon-tipped catheter to open blocked arteries. A critical part of a new treatment technique that Kapur and his colleagues developed called the “Door-to-Unload” approach, the pump—which, at less than a quarter inch in diameter, is the smallest of its kind in the world—supports the heart by taking over its task of pumping blood to the rest of the body. This allows the heart to recover faster and increases its receptivity to treatment. “The combination [seems] to reduce the amount of heart damage sustained by the heart attack,” Kapur says. “As a result, that patient is less likely to potentially develop heart failure.”
The Door-to-Unload’s pivotal clinical trial, which is actively enrolling 700 patients across the U.S. and Europe, is set to conclude in the next two to three years. Should the trial succeed, the technique could be hugely important for heart attack patients. Not only can it increase the likelihood of patients being discharged from the hospital earlier, in better condition, and with a smaller chance of heart-damage-related readmission, it can also prevent the onset of heart failure—and cut down on the $31 billion spent annually in the U.S. to treat it. “If the trial is positive,” Kapur says, “it suggests that [this technique] could change a lot [in] healthcare economics and the overall trajectory of these patients’ lives.” —Makena Gera
The Alzheimer’s Tracker
From distinguished doctor to published novelist to Hollywood film director (yes, really), Howard Weiner has worn many hats throughout his decades-long career. And now, after nearly 20 years of intense research, the codirector of the Ann Romney Center for Neurologic Diseases at Brigham and Women’s Hospital adds another major entry to his eclectic résumé: spearheading the creation of a pioneering new nasal vaccine for Alzheimer’s disease. The vaccine, which—as of December—is being tested in trials with human patients at BWH for the first time, represents an exciting advancement in battling a devastating illness for which there are currently “no good treatments,” Weiner says. “Alzheimer’s is one of the major diseases of our modern era. What we’re doing could be, really, a game-changer.”
Designed to hinder and prevent the development of the disease, which affects more than 6 million Americans, the vaccine utilizes a medication known as Protollin. If effective, the therapy—administered in two doses via a nasal spray—equips a patient’s immune system to rid the brain of Alzheimer’s-causing protein clusters known as amyloid-beta plaques. While Protollin’s usefulness in combatting these plaques in humans has yet to be determined, Weiner and his colleagues have reason to be optimistic; the drug decreased (and prevented) their accumulation, while also improving memory, in mice. “I think it has great potential,” the neurologist says.
We’ll soon find out. Phase one of the Brigham’s clinical trial, which involves 16 Alzheimer’s patients ages 65 to 80, is expected to conclude sometime this summer. If the vaccine is proven to be safe and effective in producing an immune response, the study will move into its second and third phases, which would examine the vaccine’s efficacy in a much larger number of patients. It’s a slow and arduous process (“Science doesn’t move forward in a day or two,” Weiner concedes), but one the doctor feels confident in. “Getting treatments for these neurologic diseases doesn’t come as fast as people would like, but we are making progress,” Weiner says. “I think there’s a lot of hope. That’s the message I’d like to give people.” —Andrea Timpano
The Diabetes Destroyer
After 100 years of research, shockingly little has changed in the way we treat Type 1 diabetes, the version of the disease that emerges in childhood and can’t be reversed with diet and exercise. Despite all of modern medicine’s bells and whistles—the smartphone apps and the high-tech pumps—patients are still trapped in an arduous cycle of monitoring their own blood sugar levels and taking insulin, often multiple times a day, for the rest of their lives. “For the patients and their families, the burden is on them. We give them a syringe and insulin and say, ‘You take care of your diabetes,’” says Bastiano Sanna, executive vice president and chief of cell and genetic therapies at Boston’s Vertex Pharmaceuticals. “That is a crushing burden.”
That is, scientists hope, until now. Vertex, a public firm valued at about $50 billion, stunned the medical community late last year when it announced a seemingly impossible result: Using a groundbreaking new therapy, it had landed on a potential cure. The treatment, based on research by Harvard scientist Dr. Douglas Melton, involves training stem cells to produce insulin in a lab, then infusing them into a diabetic’s liver. The first patient, a 64-year-old mailman from Ohio with a particularly severe case, found that just three months after receiving the treatment his insulin use dropped by 91 percent. By December, he was telling Good Morning America that he felt like he had “another chance at life.”
Vertex believes he won’t be the last. Further clinical trials, likely over several years, are needed. But the treatment has the potential to be revolutionary.
Still, there are obstacles. Currently, the procedure must be paired with immunosuppressives to keep the body from rejecting the cells, which, given the drugs’ harsh effect on the body’s immune system, is far from ideal. It’s also not clear how long these lab-grown cells work, or what side effects they might produce.
And yet, for the first time in a century, a cure may actually be on the horizon for the millions of diabetics worldwide. What’s more, it would also be a giant leap forward in harnessing the power of stem cells, long thought to be the key to solving some of the body’s most bedeviling problems, from spinal cord injuries to Parkinson’s. “It’s been talked about for decades,” Sanna says. “Nobody has actually done it in the clinic the way we have.” —Spencer Buell
Photo via ViaFrame/Getty Images
The Heart-Saving App
These days, there’s an app for just about everything—and now, thanks to Boston biotech firm Biofourmis, that includes treating heart failure.
Called BiovitalsHF, the first-of-its-kind software seeks to improve the quality of care for the more than 6 million adults in the U.S. who suffer from the condition. For these patients, the biggest treatment challenge is receiving the appropriate dosage of a complicated cocktail of medications, known as guideline-directed medication therapy (GDMT). With GDMT, medications are prescribed in escalating amounts, a process known as titration, based on health factors individual to each patient. The approach requires extensive, consistent doctor visits and repeated trial and error. “Most of the time, patients miss those regularly scheduled visits, or go to their primary care doctors rather than specialists,” says Biofourmis CEO and founder Kuldeep Singh Rajput. This severely decreases a clinician’s ability to monitor symptoms, heart rate, and blood pressure readings, as well as account for concurrent medications and their effects. The BiovitalsHF platform, though, “has the ability to capture [and analyze] all of these” factors in real time, Rajput explains, enabling physicians to deliver better care.
The app is composed of multiple parts: a sensor that, when worn on the upper arm, tracks a patient’s vital signs; a companion app that allows patients to report their symptoms, manage medications, and access educational content; and a clinician-facing analytics engine that makes quick treatment recommendations. For heart failure patients—less than one percent of whom receive the optimal medication dosage through traditional treatment methods—the potential benefits are life-changing. More than just an improvement in treatment efficacy, the app grants access to around-the-clock support, plus the ability for patients to manage their condition from home.
So far, the results speak for themselves. After the success of the app’s first trials in 2019 and 2020, BiovitalsHF—which, as of last July, has raised roughly $145 million in funding—received a Breakthrough Device Designation from the FDA in July 2021. Rajput says he expects the app to be fully approved within the next year—a real milestone in the world of cardiology. Digital therapeutics are already commonly used to treat other conditions, such as substance abuse disorders and diabetes, the doctor says. “[But BiovitalsHF] is the first software-based therapy for managing heart failure patients with actual treatment effect.” —M.G.
The Prescription Video Game
What if instead of taking your medicine, you could play it? That’s the new and downright radical vision driving Boston-based Akili Interactive, which in 2020 became the first-ever company to get FDA clearance for a prescription video game. Called EndeavorRx, it’s designed to be a treatment for inattention that patients, primarily kids with ADHD, actually like. “This is the first class of prescribe-able medicine that can deliver something that they enjoy, that they feel excited about, that isn’t stigmatized,” says CEO Eddie Martucci. “Beyond that, it can actually adapt. It can change. And unlike every other medicine, it can get better.”
Research has shown that the game, which asks players to complete tasks such as guiding a spaceship through an obstacle course, can help train a patient’s brain to better control cognitive and motor functions while ignoring distractions. But the real secret sauce can be found behind the scenes, where the game’s proprietary algorithm toggles difficulty up and down to provide precisely the right amount of stimulus for the patient—be they an uncoordinated youngster or a seasoned gamer—all while collecting valuable data that can help doctors track progress in real time.
Of course, a video game with medical benefits is a tough sell. (For prescribers, at least; recent data shows that venture capital investment in similar digital therapeutics grew nearly 900 percent from 2015 to 2019.) But Akili’s FDA clearance—which distinguishes the company from the Nintendos and Activisions of the world, who are prohibited by law from claiming that their products treat disease—is helping to win over doctors who might otherwise raise their eyebrows at the concept that kids can get better by gaming. “Frankly, in some of these areas, [physicians] have been burned by snake oil, because there’s just a lot of crap for the brain that’s existed in the consumer world,” Martucci says. “So there’s some skepticism, but that’s a very small fraction [of doctors].”
As for the big picture, Akili says, its strategic license to treat kids with ADHD is just the beginning. The company hopes millions of Americans with all sorts of cognitive disorders, which run the gamut from dementia to traumatic brain injury, will begin taking downloadable prescriptions in the not-so-distant future. “I think there’s so much possibility here, when you think about how technology interacts with the brain,” Martucci says. “We intend to grow our technology base pretty dramatically over the next few years.” —S.B.
Among other roles, Dr. Matthew Carty heads Brigham and Women’s Lower Extremity Reconstruction Program. / Portrait by Webb Chappell
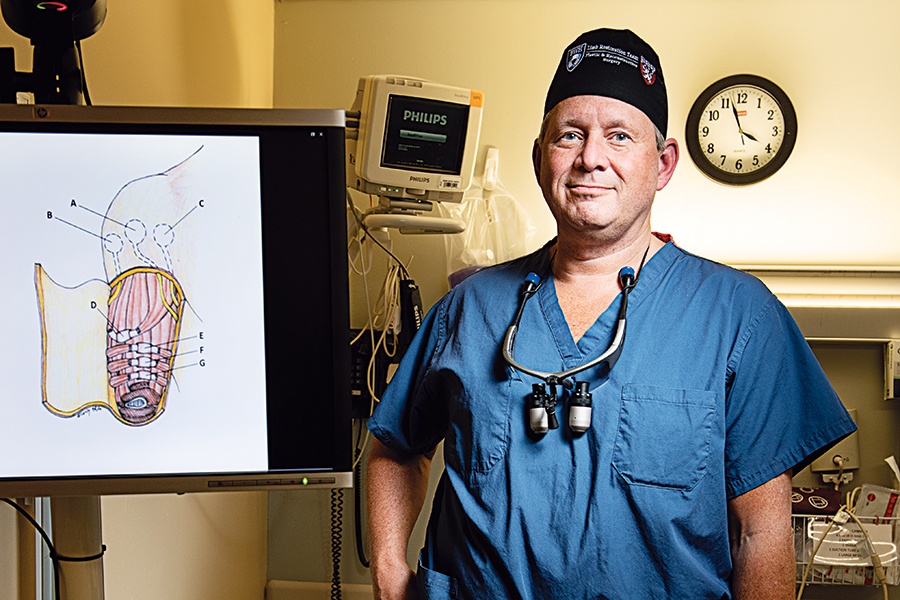
The Reconstructive Surgery
It’s not every day that scribbling on a napkin leads to a major medical breakthrough. But that’s precisely the outcome Matthew Carty and Hugh Herr set in motion while dining (and doodling) at a Boston restaurant some 10 years ago.
It was then that the pair—Carty, a specialist in reconstructive surgery at Brigham and Women’s Faulkner Hospital, and Herr, an MIT professor and acclaimed prosthetics inventor—laid the groundwork for a promising amputation procedure known as the AMI, short for the Agonist-Antagonist Myoneural Interface. In a traditional amputation, the link between the limb’s muscles—which normally work together—is severed, as is the brain’s ability to communicate with them. With an assist from those early napkin drawings and future brainstorming sessions, Carty and Herr ultimately realized they could potentially repair those connections by using matter from the amputated limb to recouple the muscles. If successful, the procedure could (among other benefits) reduce what’s known as “phantom pain” in the missing limb, while also better equipping the remaining appendage for advanced
prosthetics—very good news, considering an estimated 185,000 Americans undergo amputation procedures each year. “The design principles were already present in the body,” Carty says. “All we had to do was figure out how to operationalize the idea.”
They did. After researchers successfully tested the theory in animals and, later, on human cadavers, the first AMI procedure—a below-the-knee amputation—was performed on a live patient in 2016. Since then, 37 AMI surgeries have been completed, thanks in no small part to the ongoing efforts of Carty’s and Herr’s teams, plus collaborators at Walter Reed National Military Medical Center. While the first implementations of the AMI procedure were performed in above- and below-knee amputations, the technique can now be utilized in above- and below-elbow operations. Patients who’ve undergone traditional amputation surgeries are eligible for the procedure, too. “It continues to be more successful than I ever thought it would be,” Carty says.
Of course, there’s still more work to be done. As prosthetic technology continues to evolve, so too will the surgery to ensure alignment. And despite the growing number of success stories, Carty says it could take years for the AMI procedure—which is still being refined and standardized—to be accepted as common practice. “We know from past experience that the adoption curve for new techniques in surgery is about 20 years,” he says. Even so, Carty remains hopeful. “If we can move the needle a little bit for the benefit of these patients, that’s why we’re all here,” he says. —A.T.
The Cancer Killer
At a moment when the mere mention of the word “virus” sounds all sorts of alarms, Replimune is here with an important reminder: Not all viruses are bad. In fact, as illustrated by the Woburn-based company’s buzzy work in oncolytic immunotherapy—a burgeoning method for cancer treatment in which viruses are used to wage war on tumors—some can be quite helpful. “Obviously, some viruses are nasty,” says CEO Philip Astley-Sparke, who cofounded the $1.25 billion biotech firm in 2015. “But others are virtuous.”
He should know. Astley-Sparke previously helmed Woburn-based BioVex (now Amgen), which developed a virus-powered therapy known as Imlygic—the first of its kind to receive FDA approval for cancer treatment. It’s that success that fuels his hope in Replimune’s so-called Ignyte studies, a series of ongoing clinical trials that—like Imlygic—use a genetically modified herpes simplex virus to target tumors in patients with late-stage skin cancers. Essentially, the virus is injected into a tumor deposit, where it replicates inside (and ultimately kills) cancer cells. If effective, the souped-up bug also helps kick into gear the body’s infection-fighting T cells, which launch an attack on un-injected tumors and protect against future cancer growth. “We get cancer because of a failure of our immune systems—either a failure to recognize a tumor as foreign or, if the tumor is recognized as foreign, the ensuing immune response is inappropriately shut down,” Astley-Sparke explains. “This technology really tries to make sure the immune system does recognize the tumor as foreign in the first place. If it does that, our bodies can deal with the cancer.”
It’s a tall order, to be sure—and Replimune, which went public in 2018, is still about a year away from announcing whether data from the trials is positive enough to warrant filing for FDA approval. But researchers are encouraged by what they’ve observed so far. In the company’s melanoma study, for instance, where patients have exhausted all other treatment options, the use of the virus has resulted in a 30-percent decrease in “disease burden,” or impact of the cancer, in a promising number of enrollees. Plus, as Astley-Sparke points out, the side effects are typically minimal. “This is a well-tolerated modality, [with] fever and soreness around the injection site for a few days, and then you can get back to your life,” the CEO says. “That isn’t the same for a lot of other cancer [treatments].” —A.T.
The Vaccine Helper
When COVID-19 hit, Moderna wasn’t the only local life sciences company that sprang into action. Ginkgo Bioworks wanted to help, too. After all, the Seaport-based firm—which engineers live cells for everything from medical treatments to food production—was well-situated for the pandemic pivot.
Recognizing the world’s dire need for mRNA vaccines, the team turned their existing foundries to the task of getting higher yields of something called a vaccinia capping enzyme—a crucial part of an mRNA vaccine that helps the human body recognize it (rather than rejecting it as a foreign item). The process involved testing various ways to produce the enzyme that Patrick Boyle, the company’s head of codebase, likens to tinkering with the beer-brewing process—changing recipes, fermentation times, and more to see what generates the best result.
What they ultimately ended up with was a version of the enzyme that increased production yields tenfold, which is awfully helpful when there’s suddenly a global desire for the stuff. And there’s a good chance COVID vaccines won’t be the only ones that can make use of the technology. “There’s a handful of other mRNA vaccines that are in clinical trials right now for seasonal viruses,” Boyle says. “It could be interesting to see how this becomes a new tool to manage those.”
At the end of the day, the mission for the Ginkgo Bioworks team hasn’t changed that much—the goal has always been “to make biology easier to engineer,” as Boyle puts it. And given rapid technology advances and the company’s seemingly limitless resources—the firm was valued at $20 billion when it went public in September—there’s plenty more opportunity to keep working on it: “This is computers in the 1980s and ’90s. What we can do with the technology is changing quickly,” Boyle adds. “I don’t think we’ll be done with this sort of thing for another decade or two at least.” —Lisa Weidenfeld
Photo via Sim3D/Getty Images
The Gene Therapy
If the word “Akouos”—the name of a buzzy Boston biotech firm—brings to mind the word “acoustic,” you’re on the right track: As company founder Manny Simons explains, it’s a Greek term meaning “to hear, to listen, to understand.” In other words, not a bad moniker for a company researching gene therapy to treat genetic hearing loss.
Gene therapy, which involves replacing a faulty gene or adding a new one to remedy an ailment, has already shown promise as a treatment for an inherited form of blindness and spinal muscular atrophy. Simons says it’s a strong candidate to treat hearing loss in part because, as you might guess, the inside of your ear is not an easy part of the body to access. “The sensory cells in the ear are encased in a bone,” he explains. Thankfully, with gene therapy, those cells would only need to be reached once: Akouos’s specific treatment—administered in one shot via a specially designed device that’s inserted into the ear canal—delivers a healthy copy of a defective gene that causes deafness from birth. The process has yielded promising results in non-human primate trials, delighting the medical community and would-be investors alike. (Indeed, Akouos raised a staggering $213 million on its first day on the Nasdaq market back in 2020.) The Southie-based firm hopes to begin testing in humans in 2022, pending FDA acceptance of its application. And since they hypothesize that the therapy will show effects within a few months, researchers could know whether it’s a viable way to address hearing loss by the end of year.
More good news: While Akouos’s treatment is designed to be used very early in life, those of us who spent much of our youth in blisteringly loud concert venues may someday be able to reap the benefits of the company’s research, too. Simons concedes the runway for that may be more of a “longer-term effort” since it requires the regeneration of sensory cells, but that it could someday be doable. Translation? This approach could eventually be available for the 470 million people worldwide who have a disabling hearing loss that wasn’t caused by genetics, from Granny right on down to construction workers. We like the sound of that. —L.W.

Photo by Vicarious Surgical
The Robot
Surgical robots have come a long way since 1985, the year the world saw its first robot-assisted operation. And yet, in an industry valued at more than $5 billion worldwide, there is always room for improvement—and competition.
Exhibit A: an innovative new device from Vicarious Surgical, an up-and-coming robotics company based in Waltham. Loosely inspired by Fantastic Voyage, a classic ’60s sci-fi flick in which a group of scientists journey through a colleague’s wounded body, the robot utilizes a number of novel features to perform minimally invasive abdominal procedures such as hernia repairs. Its two arms, outfitted with 28 sensors each and specially built to mimic a surgeon’s movements, offer an unusually wide range of motion. The robot’s swivel-friendly camera, meanwhile, provides 3-D, 360-degree views—unheard of in currently used models. The cherry on top? Unlike other minimally invasive techniques that require three to five incisions, the Vicarious Surgical device—wielded by a doctor via a set of hand controllers—requires just one cut, which clocks in at less than the size of a dime. “We essentially make the surgeon feel like they’ve been beamed inside their patient and can operate from the inside out,” says CEO Adam Sachs, who cofounded the company alongside collaborators Sammy Khalifa and Barry Greene in 2014.
While Sachs says the group is still more than a year away from pursuing FDA approval for the robot, the fast-growing company, which went public in September 2021, has already drummed up significant interest. In 2019, the Vicarious Surgical device became the first surgical robot to earn a Breakthrough Device Designation from the FDA—a fact that, no doubt, impressed firm backers such as Bill Gates and Yahoo founder Jerry Yang. The firm’s work, which could prove to be stiff competition for leading developers including California-based Intuitive Surgical, has caught the attention of healthcare institutions, too: Sachs says Vicarious Surgical has partnered with major hospital chains both here in the U.S. and abroad. “After seven, eight years [of work], one of the things I’m most excited about is that we’ll be bringing the robot to market soon,” Sachs says. “We’re going to get to see its impact on surgeons and patients. I’m really excited about that.” —A.T.


